Ancient
History
The
Hadean Eon
During the Hadean eon, which represents the first 800 million years of
the Earth’s life (between about 4600 and 3800 million years), the heat released
by the collisions with planetesimals was enough to keep
molten the surface of the planet. As the frequency of the collisions decreased, the crust gradually solidified, until the
most ancient known rocks were formed.

Hadean eon: lava
landscapes (Bernhard Edmaier)
The
Archaean Eon
It was during the following eon, the Archaean, that life arose for the
first time (at least 3600 million years ago).
Initial Conditions
In the beginning, the Earth must have displayed a huge density of craters and it would be covered by an atmosphere very rich in carbon dioxide, with some
nitrogen and traces of sulphidric acid and hydrogen. It’s possible that there
was also some methane and ammonia.
The fact that the Sun was 25%
colder in this epoch was compensated by the curtain of greenhouse gases that covered Earth, namely the
carbon dioxide and the water vapour. Because the Earth, at that time, still
preserved a large proportion of radioactive atoms (generated in the supernova that preceded the formation of the solar system), the
volcanic activity was 3 times higher than today.
It’s also probable that, as the Earth cooled, an unusually high rainfall
occurrence would have created the oceans or reinforced some previous oceanic volume.
These oceans were kept liquid due to the high atmospheric pressure, because the
temperatures were well above 100 ºC (373 ºK). They were well loaded with iron
and oxygen-reductor composites (which absorb it and prevent its free existence)
such as chemical species rich in sulphur and nitrogen.
The hydrogen was produced from the reactions between the ferrous iron
existing in the rocks and the water, being these reactions encouraged by the
intense vulcanism. The existence of hydrogen opposed to the appearance of free
oxygen in the atmosphere (because it tended to react with it to form water
molecules) and favoured the accumulation of chemical products that were important
for life. If the process of hydrogen release (and subsequent escape to space, given the fact that it
is a very light gas) would have continued indefinitely, this could have driven
to the exhaustion of the oceans, as it happened on Venus.
Gaia and the First Organisms
It’s quite possible that life could have had an important role on the
preservation of the oceans, because it supplied the atmosphere with the oxygen
that combined with the hydrogen to form water molecules, which prevented the
hydrogen from escaping to space.
At the same time, the production of oxygen meant that the other
component of the carbon dioxide consumed by the first photo-synthesizers (the
carbon) wouldn’t have been given back to the atmosphere. This way, a large
proportion of the carbon that previously existed there was buried in the rocks.
As It All Emerged
It’s possible that the first organic molecules (based on carbon) were
synthesized in the interstellar space and brought to Earth by
the comets that collided with it. Some
scientists even advocate that these objects could have also supplied our planet
with the water presently existing on it.
It may also be true that the chemistry and the initial conditions on the
Earth’s surface (in the atmosphere, close to submarine volcanos or anywhere
else) would have provoked the appearance of complex organic molecules.
In the aquatic environment there would have been formed, from the
primitive organic molecules, the aminoacids (from which the proteins are made of),
the nitrogen bases and the sugar (being both fundamental foundations for the
genesis of nucleic acids, or in other words, the RNA and DNA, responsible for
the transmission of genetic characters between generations). A reproduction
mechanism subjected to mutations would have been then established.
Simultaneously, from the phosphates and fat acids they would have been formed
the membranes, which were important for the generation of the first cells,
because their existence would be impossible without the solid (consistent) and
isolating support provided by them.
The First Organisms
The food of the first cells would have consisted on organic chemical
materials that abounded in the oceans, as well as on the corpses of the less
successful competitors.
After some time, these supplies of energy and raw materials may have
started to become scarce and that provoked the emergence of organisms able to
produce their own food through photosynthesis (the capturing of solar energy in
order to produce chemical reactions that split the links that bound the oxygen
to the hydrogen (in H2O - water) and to the carbon (in CO2 –
carbon dioxide)). These bluish-green coloured organisms are known as
cyanobacteria and would have emitted some oxygen, although the wealth in reducer
chemical elements kept it in quite low proportions. The regular addition of
this element to the atmosphere and the subsequent exhaustion of the elements
that reduced it would, however, further enable its existence in large
quantities in a much later epoch.
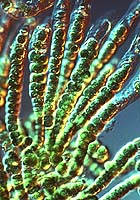
Colony of cyanobacteria
(David R. Madison)
The methanogenes were decomposers of organic products that were also
present inside this primitive biosphere. They got materials and energy through
rearrangements of the molecular products manufactured by the producing
organisms. Given the absence of oxygen they couldn’t, however, digest the
cyanobacteria directly. The methanogenes were responsible for the production of
CO2 (carbon dioxide) and CH4 (methane), which are greenhouse gases.
The abundance of cyanobacteria destroyed the carbon dioxide layer that covered
the Earth, although this molecule was continually replaced by the intense
vulcanism. The existence of the methanogenes, which gave the carbon dioxide
back to the atmosphere, was then essential for keeping the thermal equilibrium
of the Earth (if CO2 had been entirely consumed, our planet would
have become frozen).
The First “Ozone” Layer
The methane (CH4) reactions, induced by the fall of the
ultraviolet rays, would have endowed the upper atmosphere with a layer that
absorbed the ultraviolet and visible radiation of the Sun, which was therefore
equivalent to the actual ozone layer. Gases like ammonia or the sulphidric acid
could have survived at the lower atmosphere due to this protective effect.
The Nitrogen
During the last phase of the Archaean, it’s identifiable an increase on
the quantity of nitrogen. It’s probable that previously most of this element
was found under the form of an ammonium ion (NH4)+, which
is abundant in the oceans. It is possible that the ferrous iron of the oceans
stole a large quantity of ammonium ions, used for producing composites of iron
and ammonia, which contained atoms of nitrogen. The decrease of the atmospheric
carbon dioxide and the use given by life to the nitrogen may have favoured the
importance of this element as a constituent of the atmosphere. The increase of
the gaseous nitrogen made the atmospheric pressure grow and, therefore,
contributed for the reinforcement of the greenhouse effect.
Archaean Landscape
During this period the Earth should be covered by an opaque and
brownish-red atmosphere, being enlightened by an orange Sun. A brown sea
(reflecting the colour of the sky) would bathe the sand beaches depleted of
shells. There were rocks with strange shapes, formed by calcium carbonate
secreted by the cyanobacteria colonies. On emerse land there were stagnated
water puddles, blotched by the green and dark bacteria. The sounds that could
be heard in such an environment were only the wind, the waves and the bubbles
of methane sparkling in the mud. Farther away from the sea, a thin layer of
life would permanently corrode the rocks and would release nutrients and
minerals into the rain-water flows.

Archaean landscape (Early
Life on Earth, S Bengtson; Earth's Earliest Biosphere, J W Schopf)
The first rain droplets may have appeared when bacteria of the
pseudomonas’ type arose over the Earth’s surface. Typically, in the absence of
solid particles functioning as anchors, the water can be over-cooled down to
temperatures of –40 ºC without, nevertheless, congealing. Those bacteria
produce, however, a macromolecule that, along with the dust raised by the wind,
worked as a solid anchor that made easy the solidification of the water as soon
as the water fell under 0 ºC (273 ºK).
The bacteria may have used it to freeze competitors or predators, to
break the hard skin of their meals or to fracture rocks.
A solidification favoured through this process would release heat, which
would make the cloud become hotter and would, therefore, favour its rising to
the higher and colder layers. This would provoke the subsequent freezing of an
additional quantity of water vapour that existed in it. At last, when the
droplets would reach a volume and weight big enough, they would fall down under
the form of rain.
The Oxygen Surrenders the Archaean
The end of the Archaean eon occurred about 2500 million years ago, when
the production of oxygen overcame the accumulation of the reducer elements,
namely those produced by the volcanos. There started to appear oxic organisms,
which were directly feeded by the products of the cyanobacteria, including the
oxygen.
The
Proterozoic Eon
During the following period, the Proterozoic, the procaryotic organisms
(bacteria, or in other words, simple structured cells without nucleus) began to
be replaced by eucaryotic organisms (cells with a complex structure and with a
nucleus).
Eucaryots
The first eucaryot may have appeared when a bacteria tried to fence in
another bacteria and, instead of digesting it, made an association with it.
The eucaryots are formed by symbiotic organelles, or in other words, communities
of smaller entities that come together in order to get better chances of being
successful at manipulating the planet’s resources. For instance, the
chloroplasts that exist in plants (which are eucaryots) descend from the
cyanobacteria.
On the other hand, the eucaryots can’t reproduce themselves through
division (because there would be the risk that some vital organelles were not
present in each descendant) and, therefore, it was invented the sexual
reproduction.
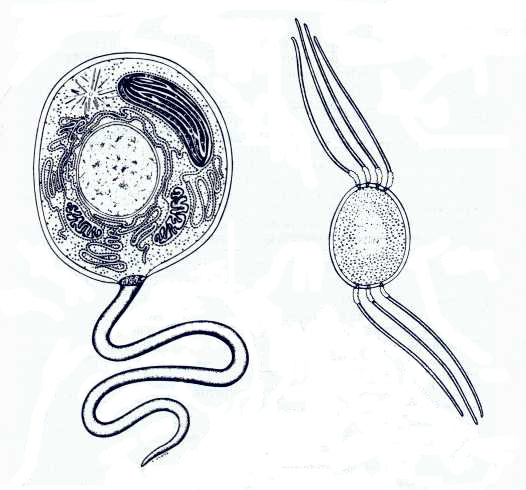
To the left: an eucaryot,
to the right: a procaryot (Christie Lyon)
The Great Glaciation
During this period, the quantity of methane diminished due to the appearance
of the oxygen as a dominant atmospheric gas. The methane was oxidised (or in
other words, reduced) by products resulting from the fall of the solar light on
the oxygen, like the hydroxyl radicals. The decline of the atmospheric methane
(greenhouse gas) would have driven the world to a glaciation 2300 million years
ago (the glaciation of Gowganda).
The Effects of the Free
Oxygen
On the other hand, the free oxygen present in the atmosphere would have
reacted with elements like carbon and sulphur, releasing acid substances into
the air. These substances would provoke an increase on the erosion of the crust
rocks, driving to the release of nutrients and, therefore, to a higher wealth
of living organisms (like the cyanobacteria), which produced more oxygen. All
this process drove to the creation of a virtuous circle.
The First Oxic Consumers
The growth of the quantity of cyanobacteria’s corpses drove to an
increase on the number of the organisms that consumed them – the methanogenes.
The organisms that were able to breathe oxygen (increasingly abundant in the
atmosphere) also gained a growing relevance, competing with the methanogenes at
the role of organic products’ consumers. Finally they ended up replacing the
methanogenes as dominant creatures. Contrarily to the methanogenes, these
organisms were directly feeded by living cyanobacteria. The falling quantity of
methanogenes would have contributed to the decrease in the proportion of
atmospheric methane.
The Stabilization
It’s thought that about 2000 million years ago the environment would
have stabilized, existing in it an already remarkable quantity of oxygen, a
lower quantity of carbon dioxide (consumed by the cyanobacteria) and a much
lower quantity of methane. The photo-synthesizers (as the cyanobacteria)
existed in large quantities, as well as the oxygen consumers, while there persisted
a small population of methanogenes. Given the decrease in the proportion of CH4
and CO2, the temperature would be lower than at the end of the
Archaean but it would be already stabilized.
Proterozoic Landscape
The appearance of the planet during that epoch was already much more
similar to its actual aspect than it was the Earth of the Archaean. The sky was
pale blue, with a cloud coverage that was eventually more dense than it is
today, the sea was greyish blue and on the beaches, besides the sand and pebble
dunes, bacteriological carpets would also extend over them. Like in the
Archaean, rocky structures built from stromatoliths’ colonies (cyanobacteria)
could be found in the sea.

The desolated landscape
of the driest desert of the world - the Atacama, in Chile – shall not differ a
lot from the aspect of the proterozoic Earth
_