Quantum
Physics
Quantum Physics is the theory that advocates that the energy is not
continuous, rather being concentrated in packs named "quanta". For
instance, the electromagnetic energy is carried by photons (light particles).
Each "quantum" (photon or any other boson) holds a quantity of energy
that is proportional to its wave frequency.
Particles
In a first distinction, the particles can be divided in 2 types: bosons
and fermions. The bosons are the field particles that can be featured as the
ones with whole numbered spins:
- 0 (they look the same whatever the side
from which they are observed);
- 1 (they only take the same aspect as we
rotate them 360║);
- or 2 (they take the same aspect if we
rotate them 180║).
The fermions are material particles (in most cases, massive ones) and
have a spin of 1/2 (they take the same aspect only after being rotated 2 times
360║)
Fermions
The fermions are subdivided in hadrons and leptons.
The hadrons are composed by quarks of different "flavours":
- up, electric charge: +2/3, mass: 310
GeV/c2;
- down, electric charge: -1/3, mass: 310
GeV/c2;
- strange, electric charge: -1/3, mass: 505
GeV/c2;
- charmed, electric charge: +2/3, mass:
1500 GeV/c2;
- beauty, electric charge: -1/3, mass: 5000
GeV/c2;
- truth, electric charge: +2/3, mass:
>22500 GeV/c2.
Each "flavour" of a quark exists in 3 "colours":
red, green and blue. Therefore, there are 6*3 = 18 varieties of quarks. Each
quark variety is corresponded by an antiquark variety, with an opposite
electric charge.
A quark can't exist isolated, because it would otherwise be
"coloured" (red, blue or green). As the strong nuclear force keeps
the particles always bound in white combinations ("colourless"), that
means that quarks have to exist in combinations of 3 (blue + green + red =
white) or 2 (blue quark + blue anti-quark = white, etc).
The triplets of quarks are called baryons and the pairs are called
mesons. The mesons, consisting in combinations of particles and anti-particles
(which tend to mutually annihilate), are unstable and give birth to electrons
and other particles. The baryons include the protons and the neutrons that
exist in atomic nuclei. A proton consists in a combination of 2 up quarks (2/3
+ 2/3 = 4/3) and one down quark (-1/3). Therefore, the electric charge of the
proton is 4/3 - 1/3 = 1. The neutron contains 2 down quarks (-1/3 -1/3 = -2/3)
and 1 up quark (2/3). So, the electric charge of the neutron is -2/3 + 2/3 = 0.
Particles can be formed from other flavours, but as they are heavier (they have
a higher mass) they decay very quickly in neutrons and protons.
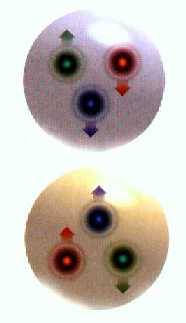
Above: neutron, below:
proton (MoonRunner Design UK)
The leptons, which are elementary particles, include:
- the electron, electric charge: -1, mass:
0,511 GeV/c2;
- the muon, electric charge: -1, mass:
106,600 GeV/c2;
- the tau, electric charge: -1, mass:
1784,000 GeV/c2.
Each one of this particles is corresponded by a neutrino (also a
lepton), each of them holding an electric charge = 0 and a very tiny mass.
There is the electronic neutrino, the muonic neutrino and the tauonic neutrino.
Bosons
The bosons, or field particles, carry each of the 4 fundamental forces
under the form of energy packs:
- the graviton is responsible for the
transportation of the gravity force, acts in an infinite range, has an
intensity 10-40 times that of the strong nuclear force and
doesn't have any mass (it moves at the speed of light). It has a spin of
2 and its existence has not been experimentally proved yet;
- the photon is responsible for the
transportation of the electromagnetic force and also acts in an infinite
range, has an intensity 10-2 times that of the strong nuclear
force and doesn't have any mass either. It has a spin of 1;
- the intermediate bosons (the W+
and W-, endowed with electric charged and with a mass of 81
GeV/c2, and the Z0, with no electric charge and
with a mass of 93 GeV/c2), are responsible for the
transportation of the weak nuclear force, act in a range smaller than 10-16
mm and have an intensity 10-13 times that of the strong
nuclear force. Since they have mass, they move at a velocity lower than
the speed of light. These 3 particles have a spin of 1;
- the gluon is the responsible for the
transportation of the strong nuclear force, acts in a range smaller than
10-13 mm and has no mass. It has a spin of 1.
Gravity, as it is analyzed in the page about relativity, is an exclusively attractive force whose intensity
depends on the mass of the bodies and on the distance that separates them.
Electromagnetism acts on electrically charged particles, as in the case
of the electrons or quarks. It may be attractive (between particles with
electric charges of opposite signal) or repulsive (between particles with
electric charges of identical signal).
The weak nuclear force is responsible for phenomena as radioactivity
(which is the disintegration of heavy nuclei - as the uranium, the thorium or
the actinium nuclei - into lighter nuclei as lead + helium nuclei + electrons +
photons) or the decay of a neutron into a proton + electron + antineutrino.
It's a force that acts on massive particles.
The strong nuclear force is responsible for the cohesion of the quarks
inside a proton or a neutron and of the protons and neutrons inside an atomic
nucleus. Its messenger particle, the gluon, only interacts with itself or with
quarks.
The Law of the Weakest
Gravity rules the Universe because its action range
is infinite and it always exerts an attractive effect, in opposition to the
electromagnetism, which also acts in an infinite range but has attractive or
repulsive effects according to the circumstances. Although the intensity of the
electromagnetic force is much higher than that of the gravity, the positive
electric charges are counter-balanced by the negative ones in the big scales
(for instance, even inside a simple hydrogen atom the positive charge of the
proton is neutralized by the negative charge of the electron). Therefore, the
big massive bodies tend to have a quite negligible electric charge.
Nevertheless, to demonstrate the innate weakness of the gravity force
compared to the electromagnetic force, it's enough to look at an apple
unfastening from the tree and falling over the floor. An apple is kept tied to
the branch of the tree by the electromagnetic forces that bind the atoms of the
apple to the atoms of the branch. It's necessary the gravity of the entire
Earth to overcome the electric force inherent to the few atoms of a modest
apple peduncle.
The Price of the Union
When 2 bodies are unified under the influence of one of these forces, the
joint mass of both is reduced, as a fraction of that mass is released under the
form of energy. For instance, an electron (negatively charged) united to a
proton (positively charged) by the electromagnetic force exists in a lower
energy condition than a freely moving electron. According to the Einstein's relativity, there is equivalence between mass and energy, so the
excessive energy that was released when the electron was caught by the proton
is also expressed as a loss of mass by these 2 bodies.
Principle
of Uncertainty
One of the pillars of quantum physics is the Heisenberg's principle of
uncertainty. According to this principle, to forecast the future position and
velocity of a particle it is necessary to be able to measure its present
position and velocity. To observe a particle it's necessary to target it with a
light beam.
If the wavelength of the beam (photon) is too long, or in other words,
less energetic, it will affect less the movement of the particle and it will be
possible to know it's velocity with a higher accuracy. However, we can't
determine the particle position with a higher precision than that represented
by the distance between 2 consecutive wave peaks (or troughs). As the
wavelength is long, that distance will be higher and, therefore, will also be
higher the uncertainty about where is the particle positioned.
The opposite will happen if we target the particle with a shorter
wavelength beam: is will provoke a bigger disruption over the particle's movement
(implying a higher uncertainty about its velocity), but it will allow us to
locate it with a higher accuracy.
Heisenberg demonstrated that the uncertainty about the position
multiplied by the uncertainty about the velocity and still multiplied by the
particle's mass can never be lower than a determined amount - the so-called
constant of Planck.
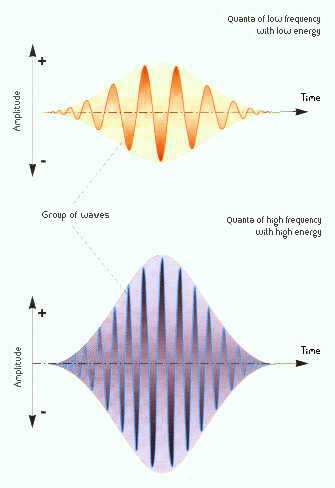
Wavelengths and
frequencies (MoonRunner Design UK)
Implications of the Principle of Uncertainty
The principle of uncertainty has deep implications in the way we
perceive the world. It's impossible to make precise forecasts about future
events, since it's not possible to accurately measure the present state of the
Universe. The quantum physics allows several possible results of an
observation, each one associated to a determined probability. Therefore, it
informs us about the probabilities of each one of the possible futures of the
world.
The Feynman's Sum of Stories
In the sequence of this idea, Richard Feynman advocated that a particle
isn't connected to a single story or pathway in the space-time. Instead, it
shall move between 2 given points following every possible pathway between
them. The probability that a particle is found in a determined place is given
by the sum of the waves associated to the stories containing that specific
place.
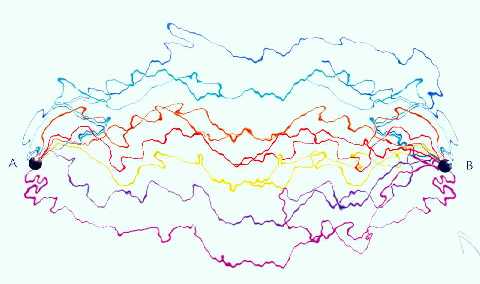
The infinity of possible
pathways, according to Feynman (MoonRunner Design UK)
The Atom
The Atom of Bohr
Even before Heisenberg formulated the principle of uncertainty (in
1926), the Danish physician Niels Bohr had defended, in 1913, that the
electrons could only occupy specific orbits around the nucleus, the closest
ones corresponding to lower energy levels and the farthest ones to higher
energy levels. We understand better the idea if we think that the less
energetic electrons are more easily and strongly dominated by the force that
links them to the protons and, therefore, are less independent from them (they
are hold by a shorter "strap").
So, an atom can only absorb or emit packs of energy (photons) with fixed
and determined wavelengths, each one corresponding to the difference between
the energy levels of 2 orbits. When an electron absorbs a determined amount of
energy (a photon with a given wavelength), it moves to a farther orbit, and the
opposite happens if it emits energy.
The Atom of Schr÷dinger
One of the main implications of the principle of uncertainty is
precisely the way the atom works. The Schr÷dinger's model postulates that the
orbits are similar to probability spheres or clouds, instead of looking like
circumferences around the nucleus. It's not possible to exactly locate the
electron that moves at a given orbit, but it's possible to calculate the
probability of finding it in this or that position.
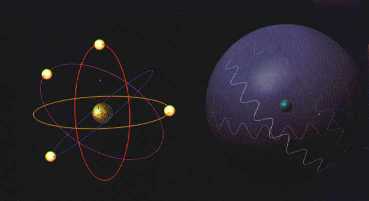
To the left: the atom of
Bohr, to the right: the atom of Schr÷dinger (MoonRunner Design UK)